NEW HAVEN, Conn., March 14, 2019 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), a clinical-stage biopharmaceutical company with a portfolio of innovative, late-stage product candidates targeting neurological and neuropsychiatric diseases, today announced that it has enrolled the first patient in a Phase 3 clinical trial assessing the efficacy and safety of troriluzole in Spinocerebellar Ataxia (SCA).
Hereditary Spinocerebellar Ataxias are rare, potentially fatal neurodegenerative disorders affecting the cerebellum. They are characterized clinically by progressive ataxia symptoms, including difficulties with balance, speech, and coordination, and are attributed to various autosomal dominant genetic mutations. Currently, there are no FDA-approved treatments and there is no cure for SCA.
Melissa Wolfe Beiner, M.D., Director of Research and Development and Medical Lead for the Ataxia development program at Biohaven commented, "Biohaven has worked closely with leading academic and clinical centers to advance this Phase 3 trial of troriluzole in this area of high unmet need." Dr. Wolfe Beiner added, "Based on learnings from our previous Phase 2b/3 clinical trial, we have enriched this trial with specific genotypes, extended the treatment period of this trial to one year, implemented the use of a modified SARA scale and increased the dose of troriluzole to 200 mg. We believe that these changes may improve the ability of the trial to more accurately evaluate troriluzole's benefit in slowing disease progression in patients with SCA."
Biohaven expects to enroll approximately 230 patients in this randomized, double-blind, placebo-controlled trial across approximately 22 sites in the United States. Researchers will evaluate the efficacy and safety of troriluzole over 48 weeks in patients with a diagnosis of SCA Types 1, 2, 3, 6, 7, 8, and 10. The primary outcome measure is the change in a patient's score on the Modified Functional Scale for the Assessment and Rating of Ataxia, a scale designed to assess the severity of symptoms in patients with SCA. Additional details about the trial [NCT03701399] can be found at www.clinicaltrials.gov.
Jeremy Schmahmann, M.D., Professor of Neurology at Harvard Medical School and Founding Director of the Ataxia Unit at Massachusetts General Hospital (MGH) added, "We are thrilled to partner with Biohaven and colleagues around the country on this exciting project. We are hopeful that this trial will provide the first convincing evidence of a medication with potential to improve the lives of our patients with SCA."
Expanded Analysis from SCA Clinical Trial BHV4157-201 and a Matched Natural History Cohort
Biohaven also announced results today of a post-hoc analysis of patients enrolled in the short-term randomization and long-term extension phase of Study BHV4157-201 [NCT02960893], an initial Phase 2b/3 randomized controlled trial of troriluzole in patients with SCA, compared to patients selected from a natural history cohort of SCA patients who were matched on multiple eligibility criteria.
The primary efficacy endpoint for this analysis was the change from baseline in the Scale for the Assessment and Rating of Ataxia (SARA) total score after 48 weeks of follow up. Patients from the natural history cohort were matched to patients from the BHV4157-201 trial on SCA Genotype (SCA1, SCA2, SCA3, SCA6), age at baseline (18 to 75 years of age), gender, SARA Score at baseline (≥ 8 and ≤ 30), and initial score on gait item of the SARA ≥ 2.
Based on analysis of covariance (ANCOVA) least square mean changes after one year were -0.34 points (representing numerical improvement with a 95% confidence interval of -0.94 to 0.26) for 81 troriluzole-treated patients versus +1.07 points (representing numerical decline with a 95% confidence interval of 0.56 to 1.58) for 112 natural history cohort patients (increasing score indicates worsening disease status). The LS Mean difference between cohorts was -1.41 points (95% confidence interval of -2.22 to -0.60) suggesting therapeutic benefits of troriluzole (p=0.0007).
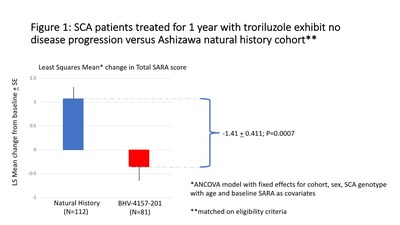
Figure 1 shows an attenuation of disease progression at one year among patients treated with troriluzole versus the natural history cohort in this post-hoc analysis. The difference in progression rates (-1.41) exceeds the minimum clinically important difference of 1.0 on the total SARA score at one year.
The natural history cohort was derived from a prospective study, conducted by the Clinical Research Consortium for Spinocerebellar Ataxias (Ashizawa, et al. 2013) and recruited from 12 ataxia clinics throughout the United States. Patients in Study BHV4157-201 were treated with 140 mg of troriluzole administered daily for one year.
Gil L'Italien, Ph.D., Head of Global Health Economics and Outcomes Research at Biohaven stated, "The findings from the post-hoc extension phase analysis of troriluzole compared to matched untreated patients from the natural history cohort are encouraging and provide further support for the potential long-term therapeutic benefit of troriluzole in patients with SCA. We are thrilled to have now enrolled the first patient in the Phase 3 study, which will more fully test the therapeutic potential of troriluzole in treating SCA over the course of one year."
About Troriluzole
Troriluzole is a third-generation prodrug and new chemical entity that modulates glutamate, the most abundant excitatory neurotransmitter in the human body. The primary mode of action of troriluzole is reducing synaptic levels of glutamate. Troriluzole increases glutamate uptake from the synapse, by augmenting the expression and function of excitatory amino acid transporters (i.e., EAAT2) located on glial cells that play a key role in clearing glutamate from the synapse. More information about trorilzuole can be found at the Company's website: https://www.biohavenpharma.com/science-pipeline/glutamate/troriluzole
About Biohaven
Biohaven is a clinical-stage biopharmaceutical company with a portfolio of innovative, late-stage product candidates targeting neurological diseases, including rare disorders. Biohaven has combined internal development and research with intellectual property licensed from companies and institutions including Bristol-Myers Squibb Company, AstraZeneca AB, Yale University, Catalent, Rutgers, ALS Biopharma LLC and Massachusetts General Hospital. Currently, Biohaven's lead development programs include multiple compounds across its CGRP receptor antagonist, glutamate modulation, and myeloperoxidase inhibitor platforms. Biohaven's common shares are listed on the New York Stock Exchange and traded under the ticker symbol BHVN. More information about Biohaven is available at www.biohavenpharma.com.
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of the Company's management. All statements, other than statements of historical facts, included in this press release, including the expected enrollment for the Company's Phase 3 trial of troriluzole, the potential results of the Company's Phase 3 trial of troriluzole in SCA, the benefits of the changes in the trial design for the Phase 3 clinical trial compared to prior trials, the potential for the Phase 3 trial to be a pivotal trial, the role of glutamate in SCA and the possible benefits of troriluzole compared to current standard of care for SCA patients, are forward-looking statements. The use of certain words, including the "believe" and "will" and similar expressions are intended to identify forward-looking statements. The Company may not actually achieve the plans and objectives disclosed in the forward-looking statements and you should not place undue reliance on the Company's forward-looking statements. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements, including uncertainties relating to the future clinical success of troriluzole. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of the Company's Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 28, 2019. The forward-looking statements are made as of this date and the Company does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
For further information, contact Dr. Vlad Coric, Biohaven's Chief Executive Officer at Vlad.Coric@biohavenpharma.com

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/biohaven-enrolls-first-patient-in-phase-3-spinocerebellar-ataxia-clinical-trial-of-troriluzole-300812397.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/biohaven-enrolls-first-patient-in-phase-3-spinocerebellar-ataxia-clinical-trial-of-troriluzole-300812397.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.


